A trusted molecule analogous to natural FVIII
This unmodified, full length rFVIII features the same posttranslational modifications found in natural FVIII, and has a primary protein structure that has been in clinical use for over 25 years.1-3

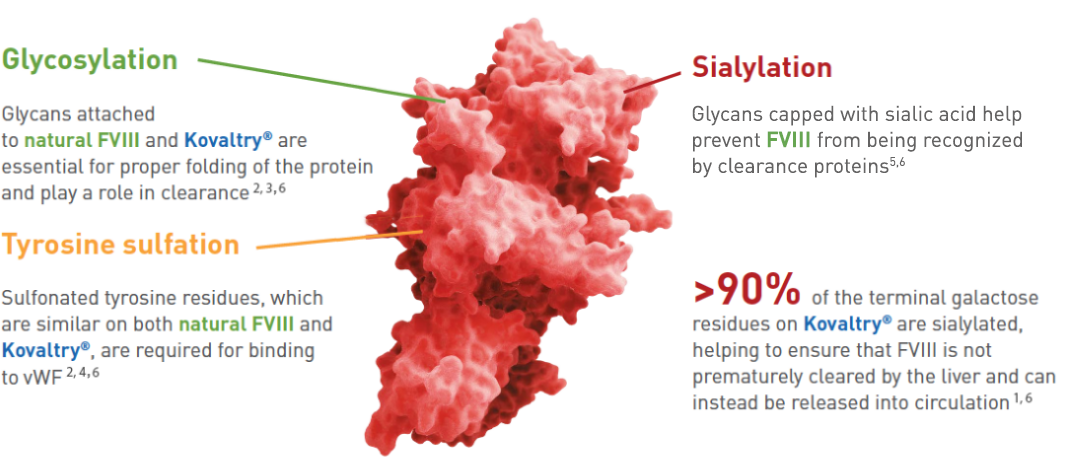
A closer look at why post-translational modifications matter
vWF, von Willebrand Factor
Recommendations
References
- Garger S, Severs J, Regan L, et al. BAY 81-8973, a full-length recombinant factor VIII: manufacturing processes and product characteristics.Haemophilia. 2017;23(2):e67-e78. Return to content
- Lenting PJ, van Mourik JA, Mertens K. The life cycle of coagulation factor VIII in view of its structure and function. Blood. 1998;92 (11):3983-3996 Return to content
- Kovaltry [Prescribing information]. Leverkusen, Germany: Bayer, 2017. Return to content
- Leyte A, van Schijndel HB, Niehrs C, et al. Sulfation of Tyr1680 of human blood coagulation factor VIII is essential for the interaction of factor VIII with von Willebrand factor. J Biol. Chem. 1991; 266 (2): 740-746. Return to content
- Bovenschen N, Rijken DC, Havekes LM, van Vlijmen BJM, Mertens K. The B domain of coagulation factor VIII interacts with the asialoglycoprotein receptor. J Thromb Haemost. 2005;3(6): 1257-1265. Return to content
- Teare J M, et al. Increased branching and sialylation of N-linked glycans correlate with an improved pharmacokinetic profile of BAY 81-8973 compared with other full-length rFVIII products. Drug Des., Dev. Ther. 2019;13:941-8. Return to content





