In a head-to-head study, Kovaltry demonstrated a superior PK profile compared to rAHF-PFM1

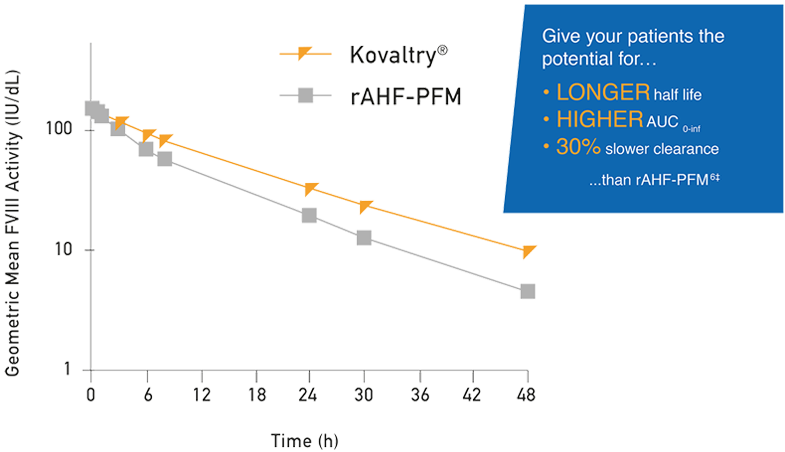
- A crossover study showed that when these two products are used in an intrapatient evaluation, significant differences are observed1*
- Each patient was randomized to a single dose of Kovaltry or rAHF-PFM – then switched to the other treatment
- At several timepoints, Kovaltry has shown a better PK profile than rAHF-PFM
Give your patients more time doing what they need to do... with Kovaltry
Kovaltry vs rAHF-PFM: time to threshold1
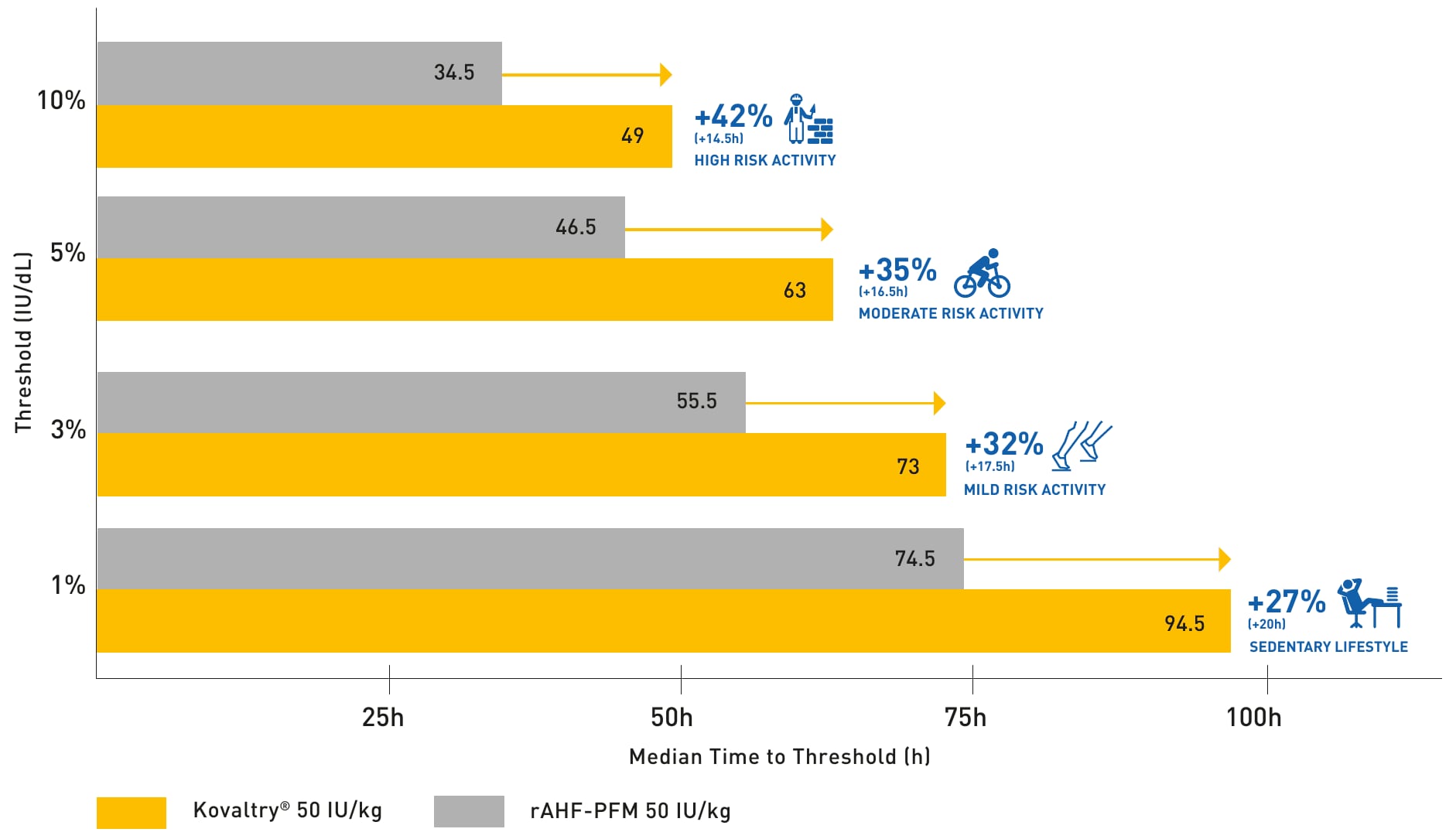
- All data were derived from a simulation of 1000 virtual patients in a popPK model, which provided an accurate description of chromogenic assay data (n=18).
- A similar trend in median time to threshold was observed for 25, 30 and 40 IU/kg doses.1
Adapted from Shah et al. 2017
*Example patient only for illustrative purposes. PopPK, population PK; rAHF-PFM, antihemophilic factor (recombinant) plasma/albumin-free method.
Recommendations
References
- Shah A, Solms A, Garmann D. Improved Pharmacokinetics with BAY 81-8973 Versus Antihemophilic Factor (Recombinant) Plasma/Albumin-Free Method: A Randomized Pharmacokinetic Study in Patients with Severe Hemophilia A. Clin Pharmacokinet. 2017;56(9):1045-1055. Return to content





