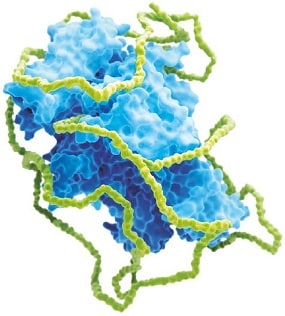
Jivi’s unique molecule design
Jivi has been designed to ensure full function of the FVIII protein, while increasing levels of FVIII over time.1

Jivi is a site-specifically PEGylated rFVIII that has reduced clearance, which leads to elevated plasma FVIII levels over time1
It features:
• 60 kDa branched (2 x 30 kDa) PEG attached to a cysteine that has been introduced into the A3 domain (K1804C), maintaining FVIII function1,2
• PEG:FVIII ratio of 1:1 (single PEGylation site)2
PEG, polyethylene glycol.
Take a look at the video below to find out more
PEG, polyethylene glycol.
References
- Mei B et al. Blood 2010; 116: 270-279. Return to content
- Ivens IA et al. Haemophilia 2013; 19: 11-20. Return to content
Learn about PEG and PEGylation of rFVIII
Get the answers to commonly asked questions about PEG, including information on PEGylation of rFVIII

What is PEG?
PEGs, short for polyethylene glycols, are synthetic, highly water soluble, inert polymers consisting of repetitive units of ethylene glycol (sometimes described as ‘ethylene oxide’), collectively known as PEG.1-3
How is PEG used in therapeutics?
In addition to a long history of use in the cosmetic and food industries,5,6 PEG is used as an excipient in viral vaccine formulations – minimizing their reliance on cold-chain supply.7,8
How is PEG used in Jivi?
Jivi has one 60-kDa PEG (2 x 30-kDa branched PEG) attached via a maleimide linker to a cysteine amino acid (K1804C) in the A3 domain introduced by site-directed mutagenesis (i.e. site-specifically PEGylated).9-11
The A3 domain was chosen through extensive testing of variants with PEG attached in each of five domains. K1804C was selected for retention of von Willebrand factor binding, coagulation function, manufacturing efficacy and improved PK vs. rFVIII-FS.11
Why is the PEG in Jivi 60 kDa?
The 60-kDa PEG size was chosen instead of the small PEG molecules (e.g. 12, 20, or 30 kDa) evaluated by Bayer because it yielded improved pharmacokinetics (e.g. longer half-life, slower clearance) while preserving full biological activity of FVIII.11,12
PK, pharmacokinetic.
References
- Baumann A et al. Drug Discov Today 2014; 19: 1623-1631. Return to content
- Ivens IA et al. Toxicol Pathol 2015; 43: 959-983. Return to content
- Zhao T et al. Biol Pharm Bull 2012; 35: 280-288. Return to content
- Fruijtier-Polloth C et al. Toxicology 2005; 214; 1-38. Return to content
- Jang HJ et al. Toxicol Res 2015; 31: 105-136. Return to content
- Braun LJ et al. Vaccine 2009; 27: 72-79. Return to content
- Pelliccia M et al. Nat Commun 2016; 7: 13520. Return to content
- Baumann A et al. Blood 2017; 130: Suppl 3674. Return to content
- Coyle TE et al. J Thromb Haemost 2014; 12(4): 488-496. Return to content
- Mei B et al. Blood 2010; 116(2): 270-279. Return to content
- Ivens IA et al. Haemophilia 2013; 19(1): 11-20. Return to content





